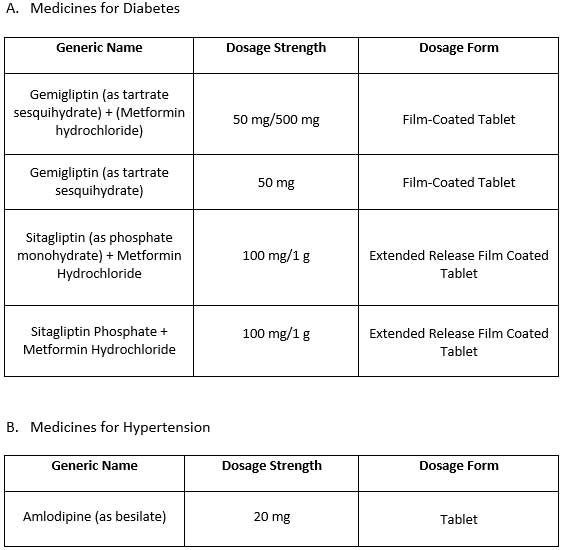
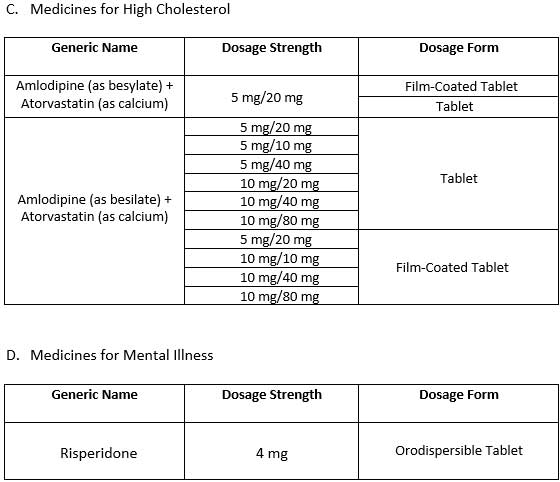
The Bureau of Internal Revenue (BIR) has released an updated list of value-added tax (VAT)-exempt medicines for diabetes, hypertension, high cholesterol, and mental illness, aiming to make essential treatments more affordable for Filipino patients.
The announcement was made through Revenue Memorandum Circular (RMC) No. 25-2025, issued by BIR Commissioner Romeo D. Lumagui Jr. on April 4, 2025.
The updated list was based on the latest publication from the Food and Drug Administration (FDA) under the Department of Health (DOH), which is responsible for identifying eligible medicines and medical devices.
These exemptions are provided under two landmark tax laws: Republic Act No. 10963, or the Tax Reform for Acceleration and Inclusion (TRAIN) Law, and Republic Act No. 11534, or the Corporate Recovery and Tax Incentives for Enterprises (CREATE) Act.
Under the CREATE Act, VAT exemptions take effect upon the publication of the FDA’s updated list, meaning qualifying drugs are immediately free from the 12 percent VAT upon release of the updated FDA notice.
“The BIR is fully aligned with the DOH and FDA in promoting health equity,” said Commissioner Lumagui.
“The tax system should never be a barrier to good health and no Filipino should have to choose between their health and their daily sustenance,” he added.
“This VAT exemption is the government’s way of making life-saving and life-sustaining medicines more accessible to all,” Lumagui said.
The exemption is expected to lower the retail prices of commonly prescribed medicines for chronic illnesses that affect millions of Filipinos, particularly those in low-income households.
According to DOH statistics, diabetes and hypertension remain leading causes of illness and mortality in the country, while access to mental health treatment continues to be a critical public health challenge.
Industry analysts estimate that VAT exemptions can cut drug prices by up to 12 percent, depending on market pricing and distribution factors.
The list of VAT-exempt medicines is available on the official websites of the BIR and FDA and is expected to be updated periodically as new drugs are approved or reclassified.