Recently, the regional offices of the department transitioned the Small Enterprise Technology Upgrading (SETUP) Program to a new phase of program implementation dubbed as SETUP 4.0.
Aside from providing firm-level assistance, the new program now ventured into industry-level assistance which include among the preparation of the S&T roadmaps. The Department of Science and Technology (DOST) Region VI is the one leading the healthcare and wellness (H&W) industry roadmap.
The H&W industry is composed of four subsectors namely, pharmaceuticals (generic drugs), herbal drugs, functional food, and food supplements and personal care products.
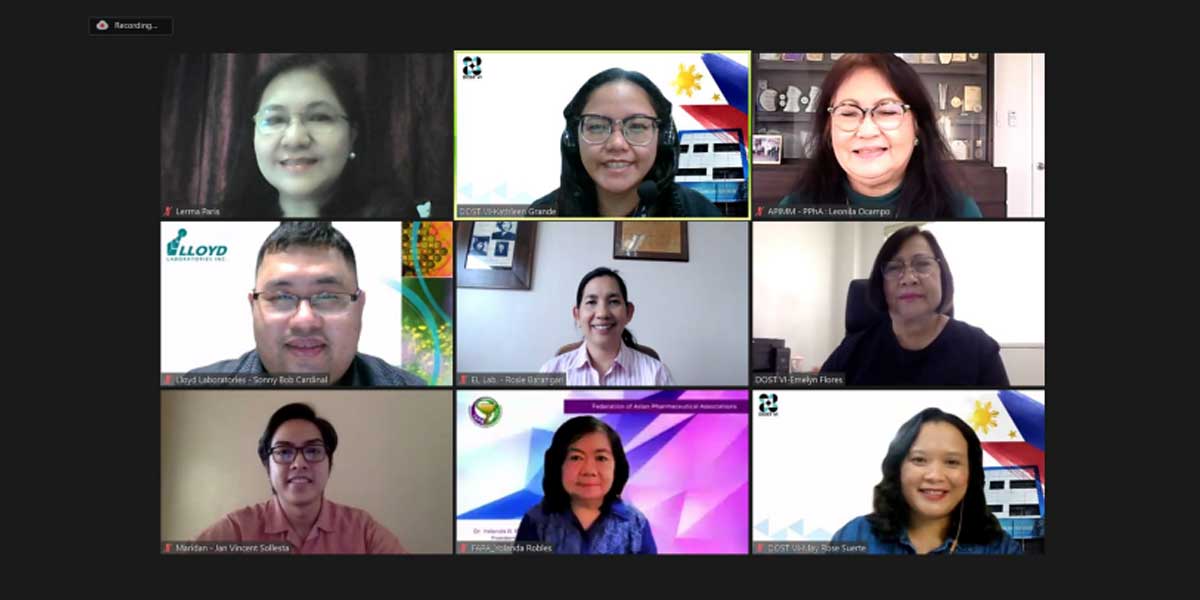
As one of the activities in crafting the roadmap, the DOST Region VI conducted numerous activities including the recent online focus group discussions to the pharmaceuticals (generic drugs) subsector.
Moderated by DOST VI consultant Ms. Lerma L. Paris, five resource speakers shared their substantial experiences and inputs about the pharmaceuticals (generic drugs) in the country.
The speakers were Vice President of EL Laboratories, Inc. Ms. Maria Rosario B. Barangan, President of the Federation of Asian Pharmaceutical Associations Dr. Yolanda R. Robles, President and Chairman of the Board of Hygieian Institute for Education, Research and Training, Inc. Ms. Leonila M. Ocampo, Business Development Manager of Lloyd Laboratories, Inc, Mr. Sonny Bob Cardinal, and Vice President of Maridan Industries, Inc., Mr. Jan Vincent N. Sollesta.
The discussions enabled the department to gather the views, experiences, insights, and opinions of the key industry players on the level of Science & Technology (S&T) utilization or adoption in their industry. One of the common issues shared was the urgent need to improve the processing of the certificate of product registrations, among others.
Moreover, it also identified factors to be considered in developing the S&T roadmaps and describe the department’s role in terms of programs and services and their implementation. (JJMoleño/Ssalazar/DOST VI-KMU)